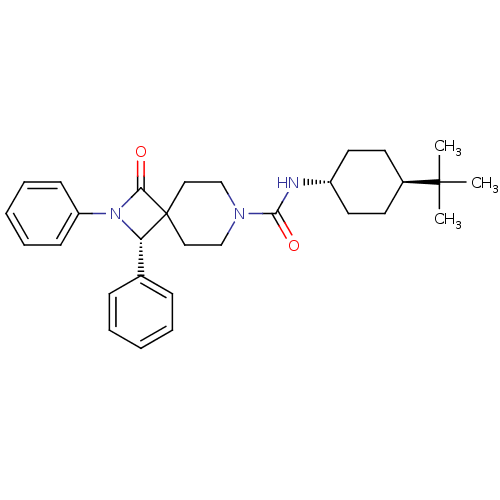
BDBM50255382 (S)-N-((1r,4S)-4-tert-butylcyclohexyl)-1-oxo-2,3-diphenyl-2,7-diazaspiro[3.5]nonane-7-carboxamide::CHEMBL481609
SMILES CC(C)(C)[C@H]1CC[C@@H](CC1)NC(=O)N1CCC2(CC1)[C@@H](N(C2=O)c1ccccc1)c1ccccc1
InChI Key InChIKey=BVDKYCVYZYRHCB-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50255382
Found 2 hits for monomerid = 50255382
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Antagonist activity at TRPV1 expressed in HEK293 cells assessed as inhibition of PMA-induced activation by FLIPR assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Antagonist activity at TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced activation by FLIPR assayMore data for this Ligand-Target Pair