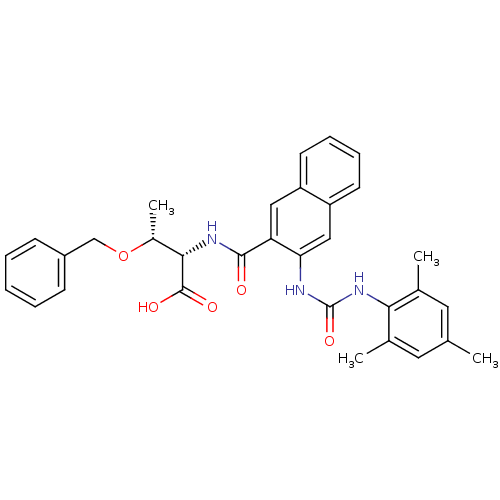
BDBM50256710 (2S,3R)-3-(benzyloxy)-2-(3-(3-mesitylureido)-2-naphthamido)butanoic acid::CHEMBL475217
SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O
InChI Key InChIKey=DYEXJFHQBKUQNA-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50256710
Found 3 hits for monomerid = 50256710
Affinity DataIC50: 16nMAssay Description:Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assayMore data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibition of liver glycogen phosphorylase A in human HepG2 cells assessed as inhibition of forskolin-induced glucogenolysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair