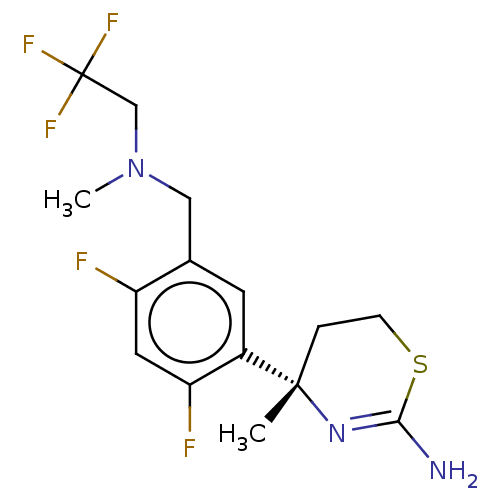
BDBM50259969 CHEMBL4104527
SMILES CN(Cc1cc(c(F)cc1F)[C@]1(C)CCSC(N)=N1)CC(F)(F)F
InChI Key InChIKey=RHXHFXVYZBDQQN-AWEZNQCLSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50259969
Found 4 hits for monomerid = 50259969
Affinity DataIC50: >8.40E+4nMAssay Description:Inhibition of BACE2 (unknown origin) by cell free assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+3nMAssay Description:Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of Cathepsin D (unknown origin) by cell free assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.02E+4nMAssay Description:Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass...More data for this Ligand-Target Pair