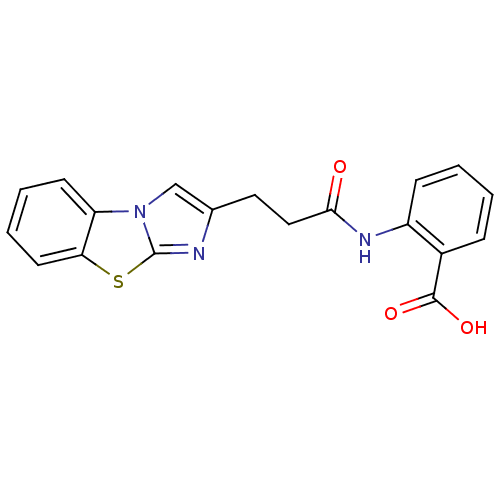
BDBM50277636 2-(3-Benzo[d]imidazo[2,1-b]thiazol-2-yl-propionylamino)-benzoic acid::CHEMBL520620
SMILES OC(=O)c1ccccc1NC(=O)CCc1cn2c(n1)sc1ccccc21
InChI Key InChIKey=FRKCVFRGEZCWGJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50277636
Found 3 hits for monomerid = 50277636
Affinity DataIC50: 31nMAssay Description:Displacement of [3H]niacin from human GPR109A expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 690nMAssay Description:Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac substrateMore data for this Ligand-Target Pair