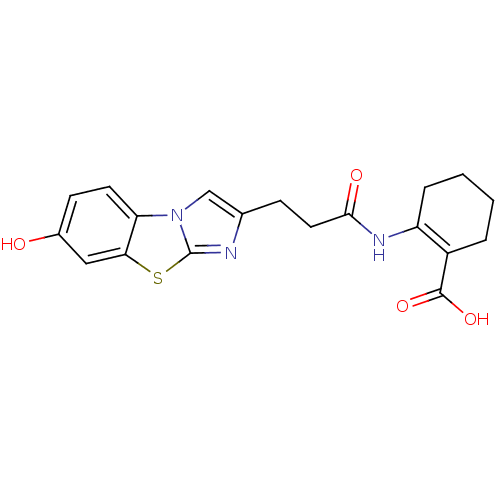
BDBM50277753 2-[3-(7-Hydroxy-benzo[d]imidazo[2,1-b]thiazol-2-yl)-propionylamino]-cyclohex-1-enecarboxylic acid::CHEMBL520513
SMILES OC(=O)C1=C(CCCC1)NC(=O)CCc1cn2c(n1)sc1cc(O)ccc21
InChI Key InChIKey=GGGKZUYCIJIKSM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50277753
Found 3 hits for monomerid = 50277753
Affinity DataIC50: 5nMAssay Description:Displacement of [3H]niacin from human GPR109A expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 76nMAssay Description:Agonist activity at human GPR109A expressed in CHOK1 cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac substrateMore data for this Ligand-Target Pair