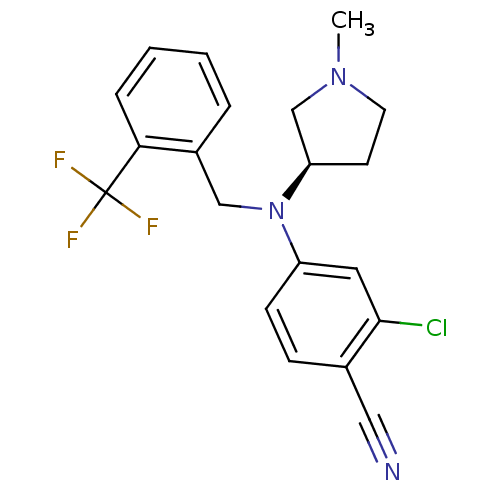
BDBM50298219 (R)-2-chloro-4-((1-methylpyrrolidin-3-yl)(2-(trifluoromethyl)benzyl)amino)benzonitrile::CHEMBL563341
SMILES CN1CC[C@H](C1)N(Cc1ccccc1C(F)(F)F)c1ccc(C#N)c(Cl)c1
InChI Key InChIKey=HMOAZJHSXXENHE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50298219
Found 4 hits for monomerid = 50298219
Affinity DataIC50: 15nMAssay Description:Binding affinity to progesterone receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Binding affinity to androgen receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 780nMAssay Description:Inhibition of human ERG by whole cell patch clamp assayMore data for this Ligand-Target Pair