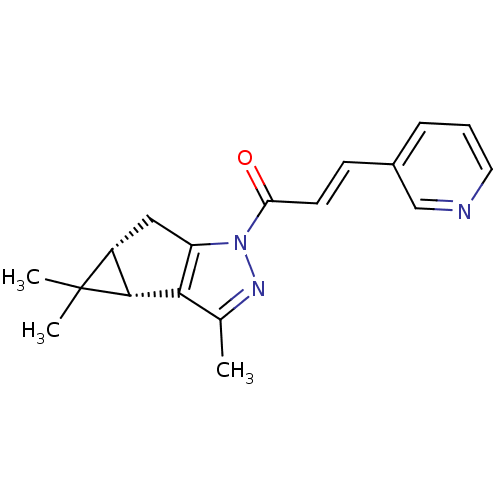
BDBM50305380 3-(Pyridin-3-yl)-1-((3bS,4aR)-3,4,4-trimethyl-3b,4,4a,5-tetrahydro-cyclopropa[3,4]cyclopenta[1,2-c]pyrazol-1-yl)-propenone::CHEMBL590384
SMILES Cc1nn(C(=O)\C=C\c2cccnc2)c2C[C@@H]3[C@H](c12)C3(C)C
InChI Key InChIKey=UXTMTCULOZQOPD-IRVACUKQSA-N
Data 4 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50305380
Found 4 hits for monomerid = 50305380
TargetSphingosine 1-phosphate receptor 1(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 17nMAssay Description:Agonist activity at human SIP1 receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetSphingosine 1-phosphate receptor 5(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 345nMAssay Description:Agonist activity at human SIP5 receptor by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetSphingosine 1-phosphate receptor 4(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: 100nMAssay Description:Agonist activity at human SIP4 receptor by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetSphingosine 1-phosphate receptor 3(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataEC50: >1.00E+3nMAssay Description:Agonist activity at human SIP3 receptor by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair