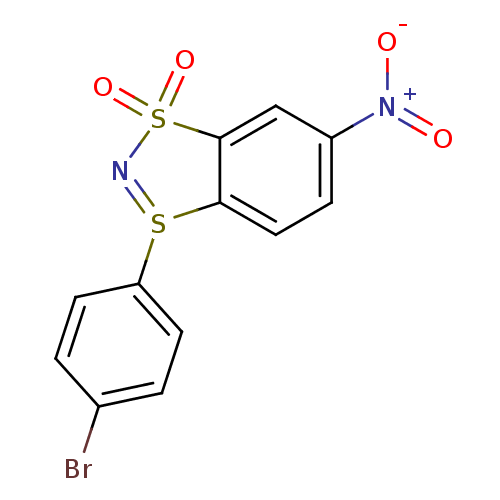
BDBM50310339 3-(4-bromophenyl)-6-nitrobenzo[1.3.2]dithiazolium-ylide 1,1-dioxide::CHEMBL605161
SMILES [O-][N+](=O)c1ccc2c(c1)S(=O)(=O)N=S2c1ccc(Br)cc1
InChI Key InChIKey=LWSWLSGAKCSXBP-UHFFFAOYSA-N
Data 7 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50310339
Found 7 hits for monomerid = 50310339
Affinity DataIC50: 1.22E+3nMAssay Description:Inhibition of LOX5 in human PMBL by enzyme immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of human recombinant COX2 expressed in insect Sf9 cells by enzyme immunoassayMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of HER2More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of COX1 in mouse RAW264.7 cells by enzyme immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of COX2 in mouse RAW264.7 cells by enzyme immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibition of human recombinant COX1 in human platelets by enzyme immunoassayMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Human)
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of HER1More data for this Ligand-Target Pair