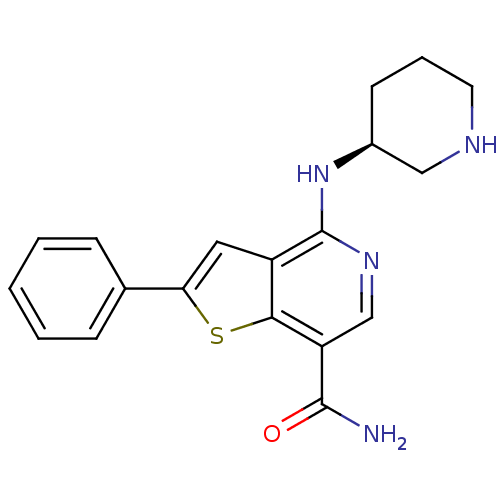
BDBM50332136 (S)-2-phenyl-4-(piperidin-3-ylamino)thieno[3,2-c]pyridine-7-carboxamide::CHEMBL1287920
SMILES NC(=O)c1cnc(N[C@H]2CCCNC2)c2cc(sc12)-c1ccccc1
InChI Key InChIKey=CXBCJDYMIUQXNZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50332136
Found 4 hits for monomerid = 50332136
Affinity DataIC50: 4nMAssay Description:Inhibition of His-tagged CHK1 after 2 hrs by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Inhibition of CHK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 26nMAssay Description:Inhibition of CHK1 in human HT29 cells assessed as abrogation of camptothecin-induced G2/M phase arrestMore data for this Ligand-Target Pair
Affinity DataIC50: 1.97E+4nMAssay Description:Inhibition of human ERG expressed in CHO cells at -80 mV holding potential by patch clamp assayMore data for this Ligand-Target Pair