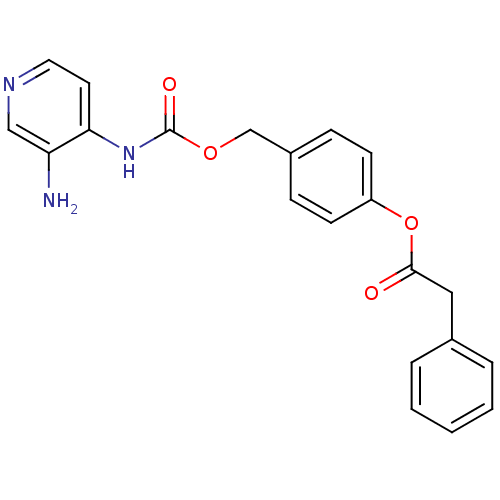
BDBM50359294 CHEMBL1928387
SMILES Nc1cnccc1NC(=O)OCc1ccc(OC(=O)Cc2ccccc2)cc1
InChI Key InChIKey=XWRKSVOQSFYXME-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50359294
Found 3 hits for monomerid = 50359294
Affinity DataKi: 3.07E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.22E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as a substrate by spectrophotometric Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.36E+5nMAssay Description:Inhibition of Electrophorus electricus Type V-S acetylcholinesterase using acetylcholine iodide as substrate by spectrophotometric Ellman's assayMore data for this Ligand-Target Pair