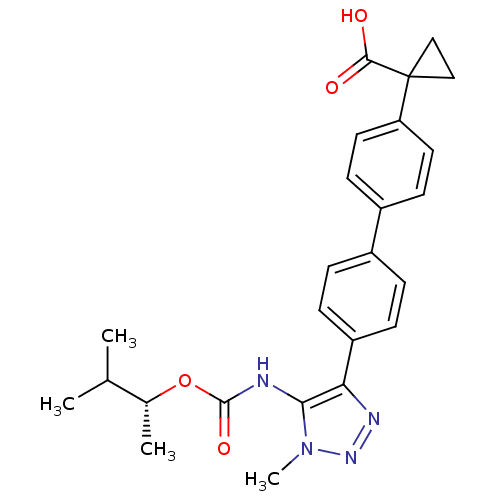
BDBM50398112 CHEMBL2182045::US9321738, 7
SMILES CC(C)[C@@H](C)OC(=O)Nc1c(nnn1C)-c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(O)=O
InChI Key InChIKey=GTLJBSMYSBXGGC-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50398112
Found 3 hits for monomerid = 50398112
Affinity DataIC50: 70nMAssay Description:Antagonist activity at human recombinant LPA1 expressed in chem-1 cells assessed as inhibition of LPA-induced intracellular calcium mobilization incu...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMT: 2°CAssay Description:Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMT: 2°CAssay Description:Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut...More data for this Ligand-Target Pair