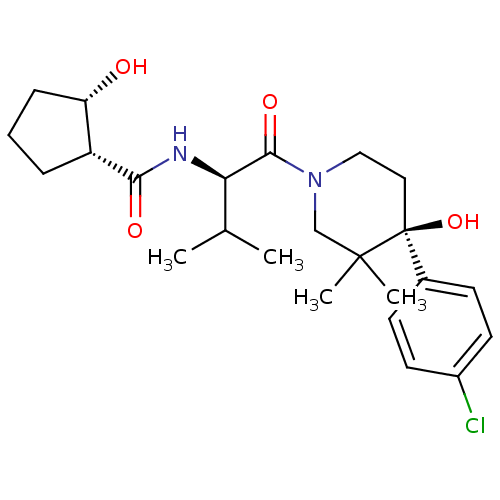
BDBM50436258 CHEMBL2398722
SMILES CC(C)[C@@H](NC(=O)[C@@H]1CCC[C@@H]1O)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1
InChI Key InChIKey=BPFQMWZMSDPOCI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50436258
Found 3 hits for monomerid = 50436258
Affinity DataIC50: 2.30nMAssay Description:Binding affinity to human CCR1More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40E+3nMAssay Description:Induction of PXR (unknown origin)More data for this Ligand-Target Pair