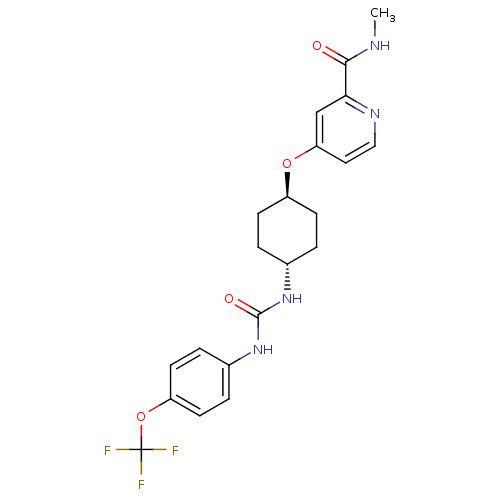
BDBM50436441 CHEMBL2397143::US9029401, 2575
SMILES CNC(=O)c1cc(O[C@H]2CC[C@@H](CC2)NC(=O)Nc2ccc(OC(F)(F)F)cc2)ccn1
InChI Key InChIKey=FLVRIUVRGJFEET-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50436441
Found 3 hits for monomerid = 50436441
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase assessed as cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxyran-2-yl)methyl] carb...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:See reference (Jones, P. D.; Wolf, N. M.; Morisseau, C.; Whetstone, P.; Hock, B.; Hammock, B. D. Anal. Biochem. 343:66-75; 2005) for sEH assay.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.5Assay Description:Inhibitor Concentration at 50% enzyme inhibition (IC50) values were calculated by quantifying the end-point ADP production from each kinase reaction ...More data for this Ligand-Target Pair