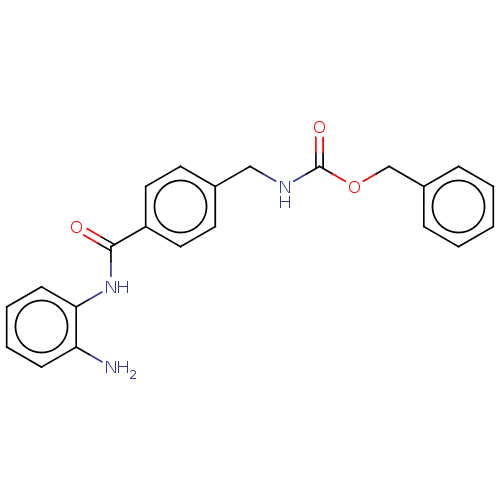
BDBM50474342 CHEMBL357268
SMILES Nc1ccccc1NC(=O)c1ccc(CNC(=O)OCc2ccccc2)cc1
InChI Key InChIKey=UZCQCEODMVDIJR-UHFFFAOYSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50474342
Found 4 hits for monomerid = 50474342
Affinity DataIC50: 544nMAssay Description:Inhibition of HDAC1 (unknown origin) using p53 residues 379 to 382 [RHKK(Ac)] as fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 613nMAssay Description:Inhibition of HDAC2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 624nMAssay Description:Inhibition of HDAC3 (unknown origin) using p53 residues 379 to 382 [RHKK(Ac)] as fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysatesMore data for this Ligand-Target Pair