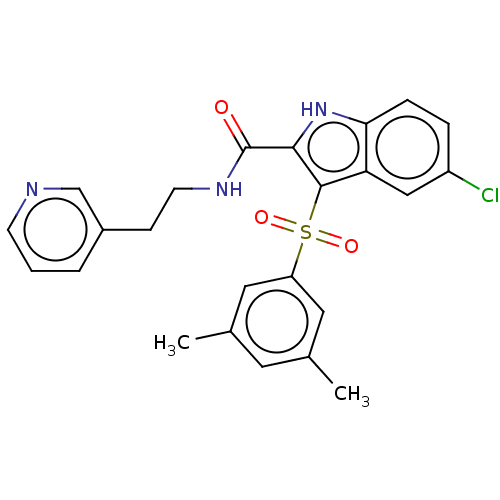
BDBM50485782 CHEMBL2163845
SMILES Cc1cc(C)cc(c1)S(=O)(=O)c1c([nH]c2ccc(Cl)cc12)C(=O)NCCc1cccnc1
InChI Key InChIKey=XFTCXYNSTYIUTL-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50485782
Found 3 hits for monomerid = 50485782
TargetReverse transcriptase protein(Human immunodeficiency virus type 1)
Sapienza University of Rome
Curated by ChEMBL
Sapienza University of Rome
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of HIV1 reverse transcriptase L100I mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrateMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus type 1)
Sapienza University of Rome
Curated by ChEMBL
Sapienza University of Rome
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of wild type HIV1 reverse transcriptase RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrateMore data for this Ligand-Target Pair
TargetReverse transcriptase protein(Human immunodeficiency virus type 1)
Sapienza University of Rome
Curated by ChEMBL
Sapienza University of Rome
Curated by ChEMBL
Affinity DataIC50: 162nMAssay Description:Inhibition of HIV1 reverse transcriptase K103N mutant RNA-dependent DNA polymerase activity using poly(rA)/oligo(dT)10:1 and [3H]-dTTP substrateMore data for this Ligand-Target Pair