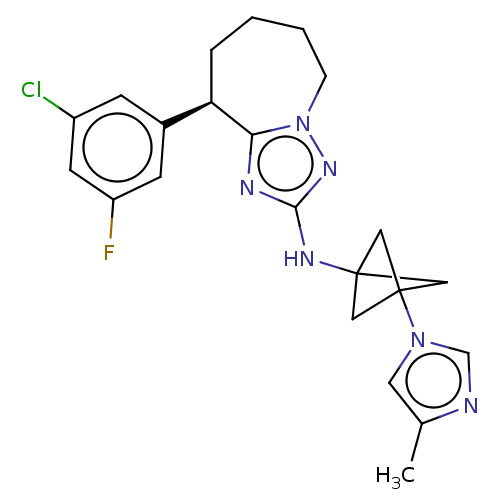
BDBM50502721 CHEMBL4435639::US11440915, Example 9
SMILES Cc1cn(cn1)C12CC(C1)(C2)Nc1nc2[C@H](CCCCn2n1)c1cc(F)cc(Cl)c1
InChI Key InChIKey=ZOYWVBKIJSBNDB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50502721
Found 2 hits for monomerid = 50502721
TargetIsoform APP695 of Amyloid-beta precursor protein [K595N,M596L](Human)
Hoffmann-La Roche
US Patent
Hoffmann-La Roche
US Patent
Affinity DataEC50: 8nMAssay Description:Human neuroglioma H4 cells overexpressing human APP695 with the Swedish double mutation (K595N/M596L) were plated at 30,000 cells/well/100 μl in...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of gamma secretase in human H4 cells over expressing human APP695 harboring K595N/M596L Swedish double mutant assessed as reduction in Abe...More data for this Ligand-Target Pair