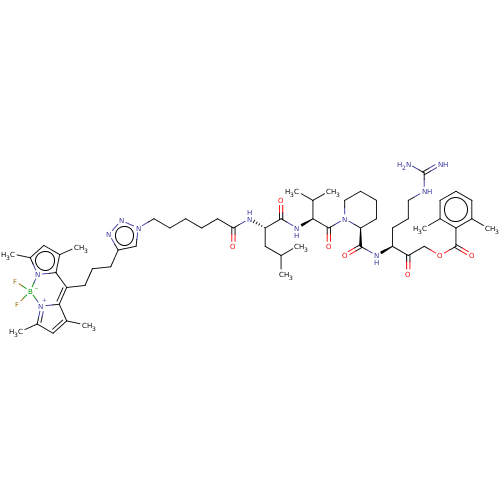
BDBM50514789 CHEMBL4564966
SMILES CC(C)C[C@H](NC(=O)CCCCCn1cc(CCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)nn1)C(=O)N[C@@H](C(C)C)C(=O)N1CCCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)COC(=O)c1c(C)cccc1C
InChI Key InChIKey=GJDIEZCMDLZTEX-UHFFFAOYSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50514789
Found 2 hits for monomerid = 50514789
TargetMucosa-associated lymphoid tissue lymphoma translocation protein 1(Human)
Ku Leuven
Curated by ChEMBL
Ku Leuven
Curated by ChEMBL
Affinity DataKi: 170nMAssay Description:Inhibition of full length wild type human N-terminal GST-tagged MALT1 catalytic domain (325 to 760 residues) expressed in Escherichia coli BL21 (DE3)...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of recombinant human cathepsin B expressed in Escherichia coli BL21 (DE3) using zRR-AMC as substrate measured after 2 hrs by fluorescence ...More data for this Ligand-Target Pair