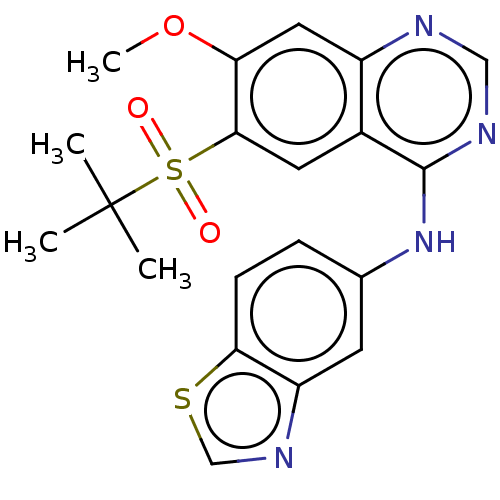
BDBM50516676 CHEMBL4473105
SMILES COc1cc2ncnc(Nc3ccc4scnc4c3)c2cc1S(=O)(=O)C(C)(C)C
InChI Key InChIKey=XNUUHYUGXNCBIN-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50516676
Found 3 hits for monomerid = 50516676
Affinity DataIC50: 4nMAssay Description:Inhibition of fluorescent-labelled ligand binding to human RIP2K preincubated for 10 mins followed by fluorescent-labelled ligand addition and measur...More data for this Ligand-Target Pair
Affinity DataIC50: 374nMAssay Description:Inhibition of RIPK2 in human whole blood assessed as reduction in MDP-induced TNFalpha production preincubated for 30 mins followed by MDP-stimulatio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG (1159 residues) expressed in CHOK1 cells at -80 mV holding potential measured after 5 mins by QPatch electrophysiology methodMore data for this Ligand-Target Pair