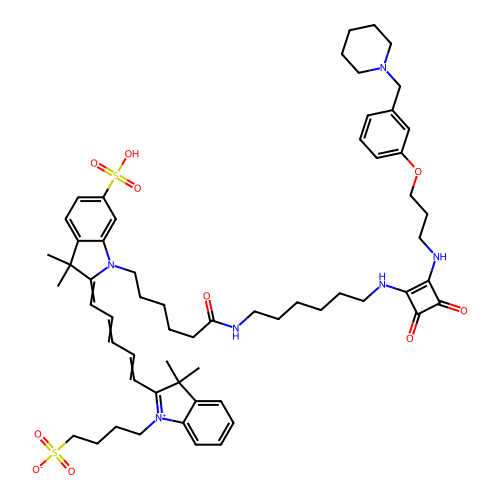
BDBM50543287 CHEMBL4641747
SMILES CC1(C)C(=CC=CC=CC2=[N+](CCCCS([O-])(=O)=O)c3ccccc3C2(C)C)N(CCCCCC(=O)NCCCCCCNc2c(NCCCOc3cccc(CN4CCCCC4)c3)c(=O)c2=O)c2cc(ccc12)S(O)(=O)=O
InChI Key InChIKey=UHGMCKHKIFEXSI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50543287
Found 2 hits for monomerid = 50543287
Affinity DataKd: 447nMAssay Description:Binding affinity to human H2R expressed in human HEK293T cells incubated for 60 mins by automated cell imaging analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.32E+3nMAssay Description:Displacement of [3H]UR-DE257 from Gsalphas-coupled human H2R expressed in baculovirus infected Sf9 cells incubated for 60 mins by scintillation count...More data for this Ligand-Target Pair