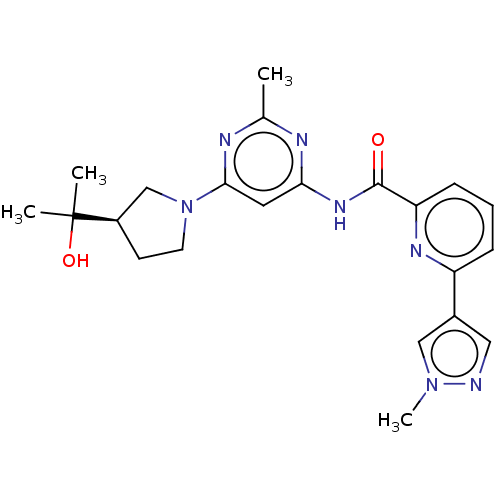
BDBM50590223 CHEMBL5171071
SMILES Cc1nc(NC(=O)c2cccc(n2)-c2cnn(C)c2)cc(n1)N1CC[C@H](C1)C(C)(C)O
InChI Key InChIKey=UPSNQHGCEYRORJ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50590223
Found 3 hits for monomerid = 50590223
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant human GST20 tagged truncated LRRK2 G2019S mutant assessed as substrate phosphorylation preincubated for 15 mins followed by...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of human LRRK2 G2019S mutant expressed in human SH-SY5Y cells assessed as inhibition of tetracycline induced LRRK2 phosphorylation at Ser9...More data for this Ligand-Target Pair
Affinity DataIC50: 255nMAssay Description:Inhibition of CLK2 (unknown origin) assessed as substrate phosphorylation using coumarin and fluorescein-labeled peptide as substrate incubated for 1...More data for this Ligand-Target Pair