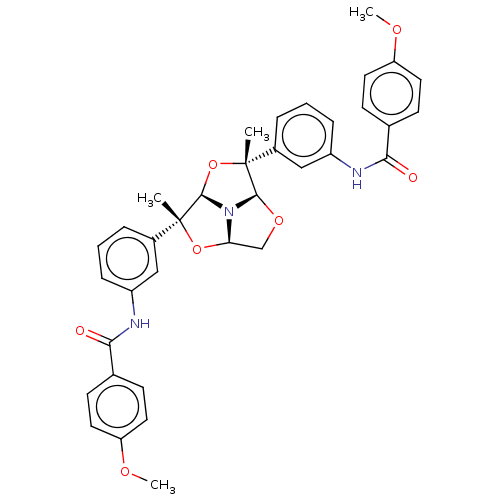
BDBM50592809 CHEMBL5172765
SMILES [H][C@@]12CO[C@@]3([H])N1[C@]([H])(O[C@]3(C)c1cccc(NC(=O)c3ccc(OC)cc3)c1)[C@](C)(O2)c1cccc(NC(=O)c2ccc(OC)cc2)c1
InChI Key InChIKey=CEFZLJAZLPOVTE-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50592809
Found 3 hits for monomerid = 50592809
Affinity DataKi: 2.20nMAssay Description:Antagonist activity at OX1R (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Antagonist activity at human OX2R expressed in CHO-K1 cells assessed as inhibition of orexin-A-induced increase in calcium mobilization preincubated ...More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Antagonist activity at human OX1R expressed in CHO-K1 cells assessed as inhibition of orexin-A-induced increase in calcium mobilization preincubated ...More data for this Ligand-Target Pair