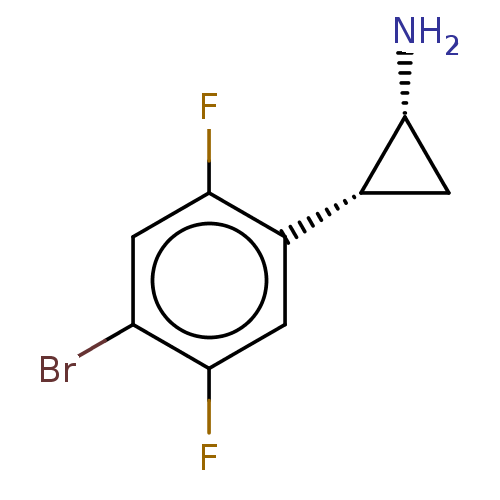
BDBM50597538 CHEMBL5206551
SMILES N[C@@H]1C[C@@H]1c1cc(F)c(Br)cc1F
InChI Key InChIKey=SGUWSJVKVPNXAI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50597538
Found 3 hits for monomerid = 50597538
TargetLysine-specific histone demethylase 1A(Human)
Riken Center For Biosystems Dynamics Research
Curated by ChEMBL
Riken Center For Biosystems Dynamics Research
Curated by ChEMBL
Affinity DataKi: 94nMAssay Description:Inhibition of LSD1 (unknown origin) (172 to 833 residues) expressed in baculovirus-infected Sf9 insect cells using K4-dimethylated H3 as substrate by...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Riken Center For Biosystems Dynamics Research
Curated by ChEMBL
Riken Center For Biosystems Dynamics Research
Curated by ChEMBL
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of human ERG by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 2(Human)
Riken Center For Biosystems Dynamics Research
Curated by ChEMBL
Riken Center For Biosystems Dynamics Research
Curated by ChEMBL
Affinity DataKi: 8.40E+3nMAssay Description:Inhibition of LSD2 (unknown origin) (26 to 822 residues) expressed in baculovirus-infected Sf9 insect cells using K4-dimethylated H3 as substrate by ...More data for this Ligand-Target Pair