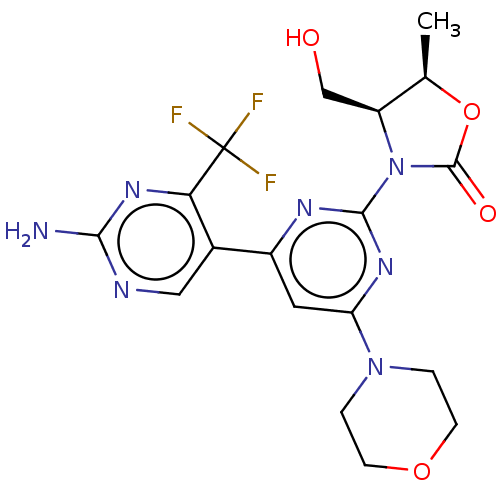
BDBM50598037 CHEMBL5203121
SMILES C[C@H]1OC(=O)N([C@H]1CO)c1nc(cc(n1)-c1cnc(N)nc1C(F)(F)F)N1CCOCC1
InChI Key InChIKey=GFZKPRZGOZINHQ-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50598037
Found 8 hits for monomerid = 50598037
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.30nMAssay Description:Inhibition of PI3Kbeta (unknown origin) using L-alpha-phosphatidylinositol as substrate in presence of ATP by Kinase Glo luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.30nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using phosphatidylinositol as substrate measured for 15 to 60 mins by TR-FRET based Adapta universal kinase ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using L-alpha-phosphatidylinositol as substrate in presence of ATP by Kinase Glo luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Rat)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibition of PI3Kalpha in rat Rat1 cells assessed as reduction in phosphorylation of AKT at Ser473 residue incubated for 1 hr by cellular assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Rat)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Inhibition of PI3Kbeta in rat Rat1 cells assessed as reduction in phosphorylation of AKT at Ser473 residue incubated for 1 hr by cellular assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate measured for 15 to 60 mins by TR-FRET based Adapta ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Mouse)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.63E+3nMAssay Description:Inhibition of mTOR in Tscl-/- null mouse MEF assessed as reduction on RPS6 phosphorylation at Ser235/236 residue by alpha-screen assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase mTOR(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.47E+3nMAssay Description:Displacement of Alexa Fluor labelled Tracer-314 from human N-terminal GST-tagged mTOR incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair