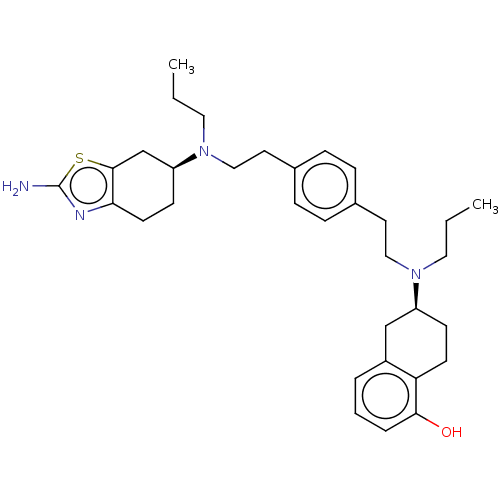
BDBM50610250 CHEMBL5267221
SMILES CCCN(CCc1ccc(CCN(CCC)[C@H]2CCc3c(O)cccc3C2)cc1)[C@H]1CCc2nc(N)sc2C1
InChI Key InChIKey=SGWUMXOWOUGLMJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50610250
Found 4 hits for monomerid = 50610250
Affinity DataKi: 2.20nMAssay Description:Displacement of [3H]-spiroperidol from rat D3 receptor expressed in HEK293 cells assessed as inhibition constant by Cheng-Prusoff equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.90nMAssay Description:Displacement of [3H]-spiroperidol from rat D2L receptor expressed in HEK293 cells assessed as inhibition constant by Cheng-Prusoff equation analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 13nMAssay Description:Agonist activity at human D2 receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding by Perkin elmer analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 13nMAssay Description:Agonist activity at human D3 receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding by Perkin elmer analysisMore data for this Ligand-Target Pair