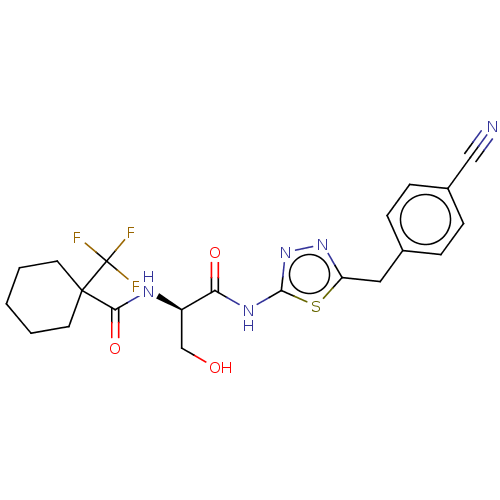
BDBM50612988 CHEMBL5286335
SMILES OC[C@@H](NC(=O)C1(CCCCC1)C(F)(F)F)C(=O)Nc1nnc(Cc2ccc(cc2)C#N)s1
InChI Key InChIKey=MLIKCSKJRFOTMN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50612988
Found 3 hits for monomerid = 50612988
Affinity DataKd: 5.80nMAssay Description:Binding affinity to recombinant human N-terminal His6-tagged USP21 (209 to 563 residues) expressed in Escherichia coli BL21 (DE3) assessed as dissoci...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human USP21 using ubiquitin rhodamine 110 110 as substrate preincubated for 15 to 20 mins followed by enzyme addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of human full length USP21 using Btn-Ahx-PNIRFLD-K(Ubi)-LPQQT-GD-amide as substrate preincubated for 15 to 20 mins followed by substrate a...More data for this Ligand-Target Pair