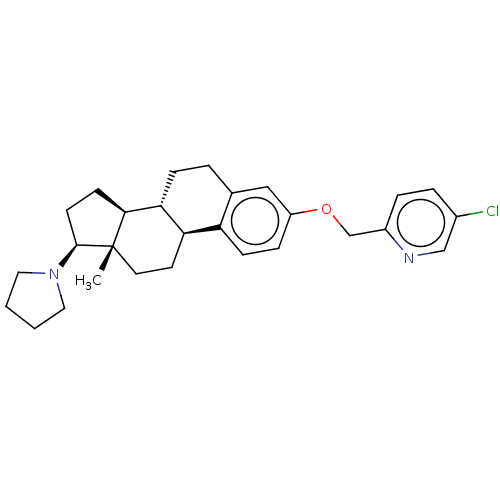
BDBM50615425 CHEMBL5271387
SMILES [H][C@@]12CC[C@H](N3CCCC3)[C@@]1(C)CC[C@]1([H])c3ccc(OCc4ccc(Cl)cn4)cc3CC[C@@]21[H]
InChI Key InChIKey=UYUQHENAGOYXKE-UHFFFAOYSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50615425
Found 2 hits for monomerid = 50615425
Affinity DataKi: 1.60nMAssay Description:Displacement of N-alpha-[methyl-3H]-methylhistamine dihydrochloride from recombinant human histamine H3 receptor expressed in CHO-K1 cells assessed a...More data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Displacement of N-alpha-[methyl-3H]-methylhistamine dihydrochloride from Sprague-Dawley rat brain membrane histamine H3 receptor assessed as inhibiti...More data for this Ligand-Target Pair