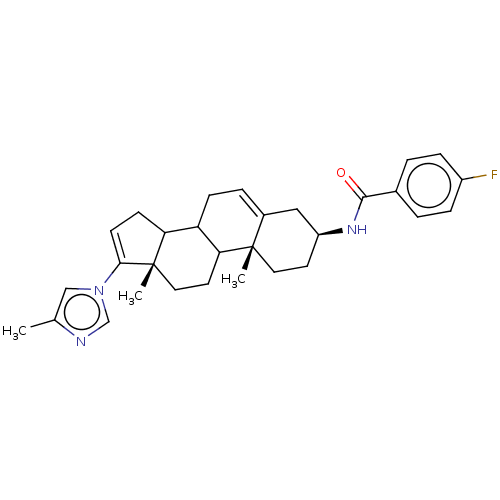
BDBM50620641 CHEMBL5400213
SMILES Cc1cn(cn1)C1=CCC2C3CC=C4C[C@H](CC[C@]4(C)C3CC[C@]12C)NC(=O)c1ccc(F)cc1
InChI Key InChIKey=JHVKBJJTUMIPIT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50620641
Found 17 hits for monomerid = 50620641
Affinity DataIC50: 80nMAssay Description:Antagonist activity at androgen receptor T877A mutant expressed in HEK293 cells assessed as reduction in DHT-induced transcriptional activation of an...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Human)
Shanghai Institute of Materia Medica
Curated by ChEMBL
Shanghai Institute of Materia Medica
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of recombinant human CYP17A1 using progesterone as substrate incubated for 5 mins in presence of NADPH by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 103nMAssay Description:Antagonist activity at ERalpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 121nMAssay Description:Antagonist activity at wild type androgen receptor expressed in HEK293 cells assessed as reduction in DHT-induced transcriptional activation of andro...More data for this Ligand-Target Pair
Affinity DataIC50: 371nMAssay Description:Antagonist activity at androgen receptor W741L mutant expressed in HEK293 cells assessed as reduction in DHT-induced transcriptional activation of an...More data for this Ligand-Target Pair
Affinity DataIC50: 390nMAssay Description:Antagonist activity at androgen receptor F876L mutant expressed in HEK293 cells assessed as reduction in DHT-induced transcriptional activation of an...More data for this Ligand-Target Pair
Affinity DataIC50: 498nMAssay Description:Antagonist activity at ERbeta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 810nMAssay Description:Inhibition of CYP3A4 (unknown origin) using testosterone as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam substrateMore data for this Ligand-Target Pair
Affinity DataEC50: 1.28E+3nMAssay Description:Induction of androgen receptor degradation in human LNCaP cells incubated for 24 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Antagonist activity at PR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Antagonist activity at GR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.08E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Shanghai Institute of Materia Medica
Curated by ChEMBL
Shanghai Institute of Materia Medica
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human ERG by patch clamp assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair