BDBM50635296 CHEMBL5512498
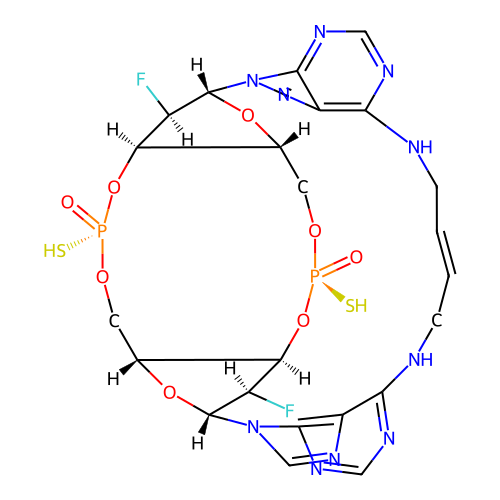
SMILES O=[P@]1(S)OC[C@H]2O[C@@H]3[C@H](F)[C@@H]2O[P@](=O)(S)OC[C@H]2O[C@H]([C@H](F)[C@@H]2O1)n1cnc2c(ncnc21)NC/C=C/CNc1ncnc2c1ncn23
InChI Key
PDB links: 2 PDB IDs match this monomer.
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50635296
Found 5 hits for monomerid = 50635296
Affinity DataKd: 40nMAssay Description:Binding affinity to recombinant human wild type STING expressed in Escherichia coli BL21 DE3 assessed as dissociation constant by Surface plasmon res...More data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+3nMAssay Description:Agonist activity at human wild type STING expressed in human THP-1 cells assessed increase in IRF3 phosphorylation incubated for 6 hrs by Western blo...More data for this Ligand-Target Pair
Affinity DataEC50: 1.20E+3nMAssay Description:Agonist activity at human STING AQ variant expressed in human THP-1 cells expressing IRF inducible SEAP reporter construct assessed as increase in in...More data for this Ligand-Target Pair
Affinity DataEC50: 2.20E+3nMAssay Description:Agonist activity at human STING HAQ variant expressed in human THP-1 cells expressing IRF inducible SEAP reporter construct assessed as increase in i...More data for this Ligand-Target Pair
Affinity DataEC50: 4.90E+3nMAssay Description:Agonist activity at human STING REF variant expressed in human THP-1 cells expressing IRF inducible SEAP reporter construct assessed as increase in i...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)