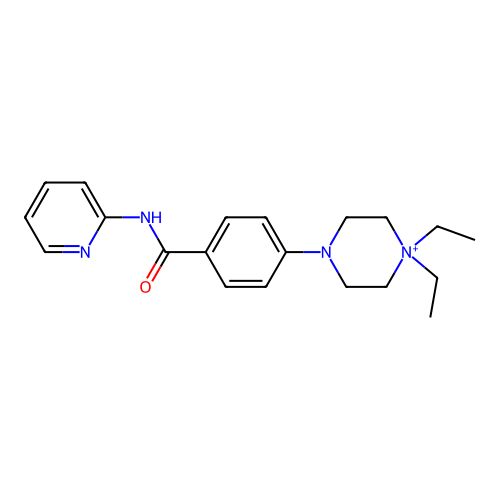
BDBM50636716 CHEMBL5532397
SMILES CC[N+]1(CC)CCN(c2ccc(C(=O)Nc3ccccn3)cc2)CC1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50636716
Found 3 hits for monomerid = 50636716
Affinity DataEC50: 1.18E+3nMAssay Description:Agonist activity at human alpha9alpha10 nAChR expressed as xenopus laevis oocytes assessed as increase in receptor desensitization measured after 120...More data for this Ligand-Target Pair
Affinity DataEC50: 1.80E+4nMAssay Description:Agonist activity at human alpha9 nAChR expressed as xenopus laevis oocytes assessed as increase in receptor desensitization measured after 120 secs b...More data for this Ligand-Target Pair
Affinity DataEC50: 2.60E+4nMAssay Description:Agonist activity at human alpha7 nAChR expressed as xenopus laevis oocytes assessed as increase in receptor desensitization measured after 120 secs b...More data for this Ligand-Target Pair