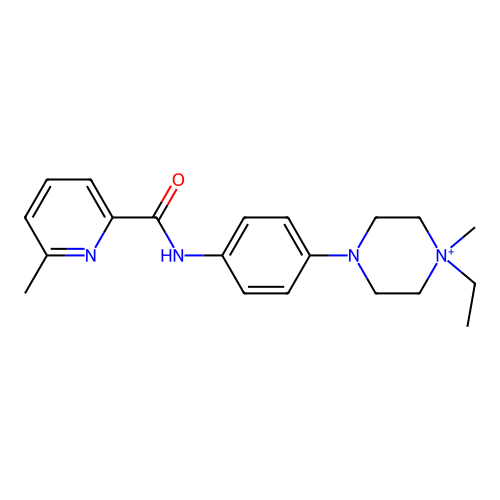
BDBM50636720 CHEMBL5558610
SMILES CC[N+]1(C)CCN(c2ccc(NC(=O)c3cccc(C)n3)cc2)CC1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50636720
Found 3 hits for monomerid = 50636720
Affinity DataEC50: 230nMAssay Description:Agonist activity at human alpha9 nAChR expressed as xenopus laevis oocytes assessed as increase in receptor desensitization measured after 120 secs b...More data for this Ligand-Target Pair
Affinity DataEC50: 990nMAssay Description:Agonist activity at human alpha9alpha10 nAChR expressed as xenopus laevis oocytes assessed as increase in receptor desensitization measured after 120...More data for this Ligand-Target Pair
Affinity DataEC50: 7.83E+4nMAssay Description:Agonist activity at human alpha7 nAChR expressed as xenopus laevis oocytes assessed as increase in receptor desensitization measured after 120 secs b...More data for this Ligand-Target Pair