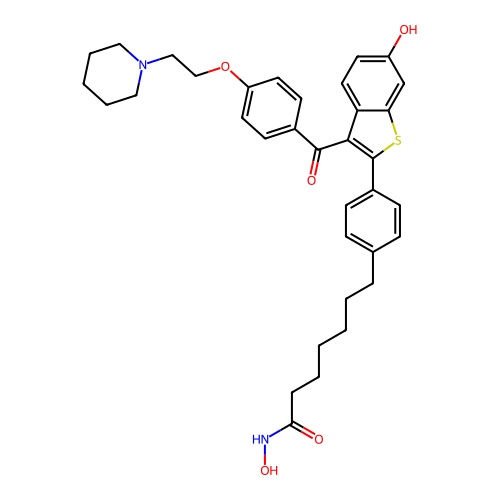
BDBM50645137 CHEMBL5590308
SMILES O=C(CCCCCCc1ccc(-c2sc3cc(O)ccc3c2C(=O)c2ccc(OCCN3CCCCC3)cc2)cc1)NO
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50645137
Found 3 hits for monomerid = 50645137
Affinity DataIC50: 333nMAssay Description:Inhibition of recombinant human HDAC6 using Ac-Val-Gly-(NAc)Lys-AMC as substrate incubated for 30 mins by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 344nMAssay Description:Inhibition of recombinant human HDAC1 using Ac-Val-Gly-(NAc)Lys-AMC as substrate incubated for 30 mins by fluorescence based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.67E+3nMAssay Description:Antagonist activity at C-terminal RLucII-fused human ER-alpha expressed in HEK293T cells co-expressing CoA-YFP-fused LXXLL coactivator motif from NCO...More data for this Ligand-Target Pair