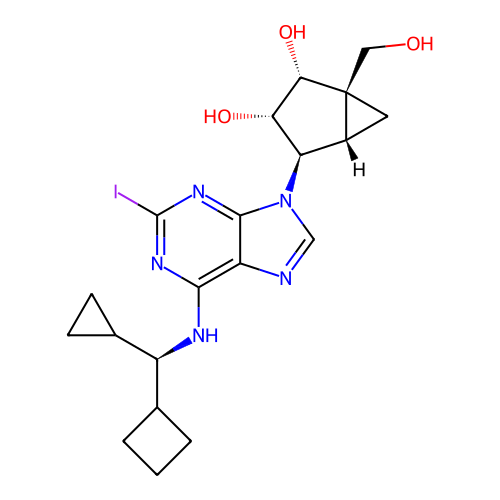
BDBM50651241 CHEMBL5631402
SMILES OC[C@@]12C[C@@H]1[C@@H](n1cnc3c(N[C@@H](C4CCC4)C4CC4)nc(I)nc31)[C@H](O)[C@@H]2O
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50651241
Found 4 hits for monomerid = 50651241
Affinity DataKi: 17nMAssay Description:Displacement of [3H]-LSD from human 5-HT2B receptor expressed in HEK cells assessed as inhibition constant incubated for 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 64nMAssay Description:Displacement of [3H]-mesulergine from human A1AR expressed in Flp-In-T-REx-293 cells assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 552nMAssay Description:Displacement of [3H]-Mesulergine from human 5-HT2C expressed in Flp-In-T-REx-293 cells assessed as inhibition constant incubated for 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity to histamine H1 receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair