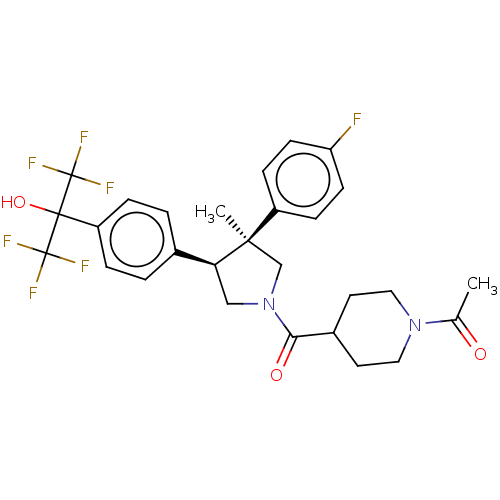
BDBM510571 US11078186, Example 25::US11078186, Example 95::rac-1-(4-((3S,4S)-3-(4-fluorophenyl)-4-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)-3-methylpyrrolidine-1-carbonyl)piperidin-1-yl)ethanone
SMILES CC(=O)N1CCC(CC1)C(=O)N1C[C@@H](c2ccc(cc2)C(O)(C(F)(F)F)C(F)(F)F)[C@](C)(C1)c1ccc(F)cc1
InChI Key InChIKey=PLEBLNIHUKCNPF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 510571
Found 4 hits for monomerid = 510571
Affinity DataIC50: 108nMAssay Description:The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 143nMAssay Description:Inverse agonist activity at RORgammat (unknown origin) expressed in Jurkat cells by reporter assayMore data for this Ligand-Target Pair
Affinity DataIC50: 311nMAssay Description:The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 1.08E+3nMAssay Description:Activation of PXR (unknown origin)More data for this Ligand-Target Pair