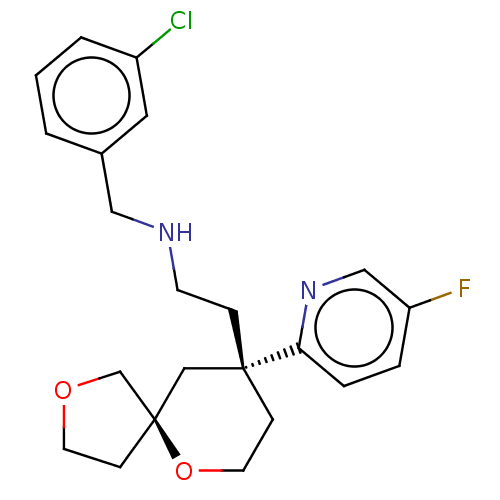
BDBM518578 US11124523, Example (+)-16
SMILES Fc1ccc(nc1)[C@]1(CCNCc2cccc(Cl)c2)CCO[C@]2(CCOC2)C1
InChI Key InChIKey=VDEAHPKUJXNLGH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 518578
Found 17 hits for monomerid = 518578
Affinity DataEC50: 0.350nMAssay Description:Agonist activity at mu opioid receptor (unknown origin) assessed as increase in cAMP level incubated for 40 mins by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataEC50: 0.5nMAssay Description:The experiment was performed using a cAMP detection kit from Cisbio (Cisbio #62AM4PEJ).More data for this Ligand-Target Pair
Affinity DataKi: 0.514nMAssay Description:Displacement of [3H]-Diprenorphine from human mu opiod receptor expressed in CHO cells incubated for 1 hr by competition binding assayMore data for this Ligand-Target Pair
Affinity DataEC50: 5.10nMAssay Description:Agonist activity at human mu opioid receptor expressed in HEK293 assessed as increase in calcium mobilization incubated for 60 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human delta opioid receptor expressed in HEK293 assessed as increase in calcium mobilization incubated for 60 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human kappa opioid receptor expressed in HEK293 assessed as increase in calcium mobilization incubated for 60 mins by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+4nMAssay Description:To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymesMore data for this Ligand-Target Pair
Affinity DataIC50: 2.28E+4nMAssay Description:To test the blocking effects of the compounds of the present disclosure on hERG potassium currents.More data for this Ligand-Target Pair
Affinity DataIC50: 2.28E+4nMAssay Description:Inhibition of human ERG expressed in CHO cells at holding potential of -80 mV by whole cell patch clamp assayMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Agonist activity at mu opioid receptor (unknown origin) assessed as beta arrestin-2 recruitment incubated for 3 days by PathHunter assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.37E+4nMAssay Description:To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymesMore data for this Ligand-Target Pair
Affinity DataIC50: 4.06E+4nMAssay Description:To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymesMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymesMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymesMore data for this Ligand-Target Pair