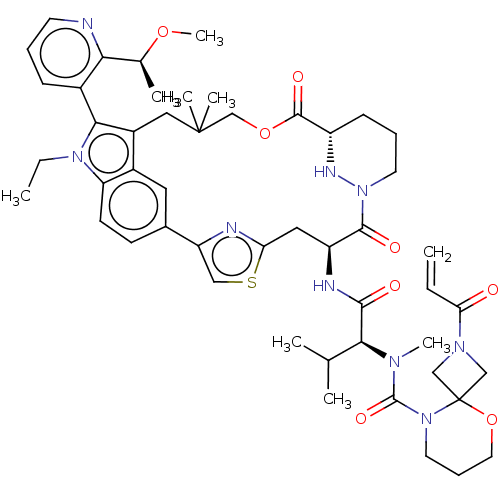
BDBM592055 Synthesis of 2-acryloyl-N-((2S)-1-(((63S,4S,Z)-11-ethyl-12-(2-((S)-1-methoxyethyl)pyridin-3-yl)-10,10-dimethyl-5,7-dioxo-61,62,63,64,65,66-hexahydro-11H-8-oxa-2(4,2)-thiazola-1(5,3)-indola-6(1,3)-pyridazinacycloundecaphane-4-yl)amino)-3-methyl-1-oxobutan-2-yl)-N-methyl-5-oxa-2,9-diazaspiro[3.5]nonane-9-carboxamide::US11566007, Example A735
SMILES CCn1c(c2CC(C)(C)COC(=O)[C@@H]3CCCN(N3)C(=O)[C@H](Cc3nc(cs3)-c3ccc1c2c3)NC(=O)[C@H](C(C)C)N(C)C(=O)N1CCCOC11CN(C1)C(=O)C=C)-c1cccnc1[C@H](C)OC
InChI Key InChIKey=MEXMPINDCBWWDY-UHFFFAOYSA-N
Data 12 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 592055
Found 12 hits for monomerid = 592055
Affinity DataIC50: 10nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ...More data for this Ligand-Target Pair