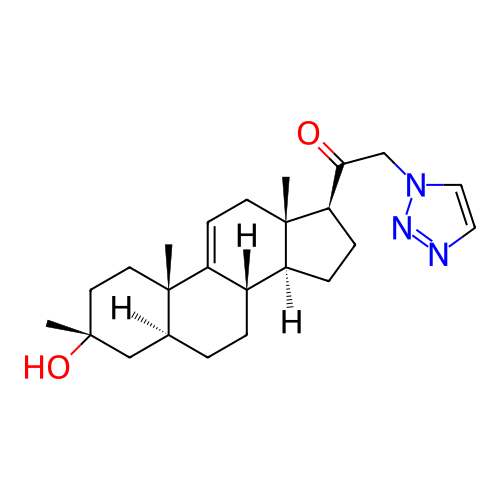
BDBM737512 Synthesis of 1-((3R,5S,8S,10S,13S,14S,17S)-3-hydroxy-3,10,13-trimethyl-2,3,4,5,6,7,8,10,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-(1H-1,2,3-triazol-1-yl)ethan-1-one (A11) & 1-((3R,5S,8S,10S,13S,14S,17S)-3-hydroxy-3,10,13-trimethyl-2,3,4,5,6,7,8,10,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)-2-(2H-1,2,3-triazol-2-yl)ethan-1-one (A12)::US20250136638, Example 6
SMILES C[C@@]1(O)CC[C@]2(C)C3=CC[C@]4(C)[C@@H](C(=O)Cn5ccnn5)CC[C@H]4[C@@H]3CC[C@H]2C1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 737512
Found 1 hit for monomerid = 737512
Affinity DataIC50: 550nMAssay Description:Briefly, cortices are rapidly removed following decapitation of carbon dioxide-anesthetized Sprague-Dawley rats (200-250 g). The cortices are homogen...More data for this Ligand-Target Pair