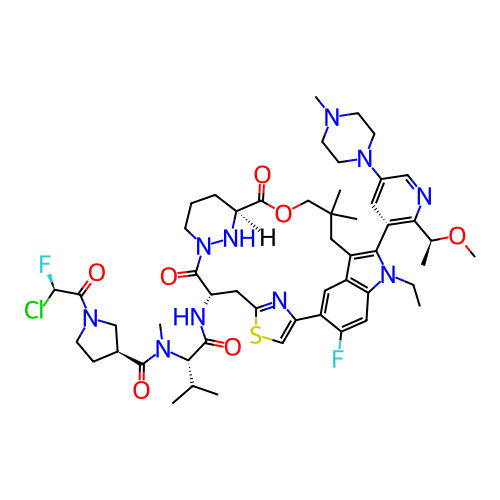
BDBM754054 (3S)-1-[(2R)-2-chloro-2-fluoro-acetyl]-N-[(1S)-1-[[(7S,13S)-21-ethyl-24-fluoro-(20M)-20-[2-[(1S)-1-methoxyethyl]-5-(4-methylpiperazin-1-yl)-3-pyridyl]-17,17-dimethyl-8,14-dioxo-15-oxa-4-thia-9,21,27,28-tetrazapentacyclo[17.5.2.12,5.19,13.022,26]octacosa-1(25),2,5(28),19,22::US20250206761, Example 32
SMILES CCn1c(-c2cc(N3CCN(C)CC3)cnc2[C@H](C)OC)c2c3cc(c(F)cc31)-c1csc(n1)C[C@H](NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H]1CCN(C(=O)[C@H](F)Cl)C1)C(=O)N1CCC[C@H](N1)C(=O)OCC(C)(C)C2
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 754054
Found 3 hits for monomerid = 754054
TargetGTPase KRas [G12C]/Serine/threonine-protein kinase B-raf/Peptidyl-prolyl cis-trans isomerase(Human)
Hoffmann-La Roche
US Patent
Hoffmann-La Roche
US Patent
Affinity DataIC50: 56nMAssay Description:In this example, TR-FRET was also used to measure the compound or compound-CYPA dependent disruption of the KRAS G12C-BRAF complex. This protocol was...More data for this Ligand-Target Pair
TargetGTPase KRas [G12V]/Serine/threonine-protein kinase B-raf/Peptidyl-prolyl cis-trans isomerase (Human)
Hoffmann-La Roche
US Patent
Hoffmann-La Roche
US Patent
Affinity DataIC50: 1.00E+4nMAssay Description:In this example, TR-FRET was also used to measure the compound or compound-CYPA dependent disruption of the KRAS G12C-BRAF complex. This protocol was...More data for this Ligand-Target Pair
TargetGTPase KRas [G12D]/Serine/threonine-protein kinase B-raf/Peptidyl-prolyl cis-trans isomerase (Human)
Hoffmann-La Roche
US Patent
Hoffmann-La Roche
US Patent
Affinity DataIC50: 1.00E+4nMAssay Description:In this example, TR-FRET was also used to measure the compound or compound-CYPA dependent disruption of the KRAS G12C-BRAF complex. This protocol was...More data for this Ligand-Target Pair