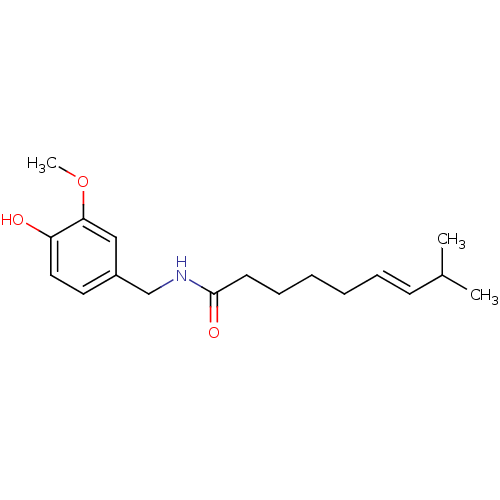
BDBM20461 (6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide::(E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide::CAP::CHEMBL294199::Capsaicin::Zostrix
SMILES CC(C)/C=C/CCCCC(=O)NCc1ccc(c(c1)OC)O
InChI Key InChIKey=YKPUWZUDDOIDPM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 59 hits for monomerid = 20461
Found 59 hits for monomerid = 20461
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 2.51nMAssay Description:Agonist activity at human TRPV1 expressed in tetracycline-stimulated HEK293 cells assessed as increase in intracellular calcium levels by fluorimetri...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 3.90nMAssay Description:Agonist activity at human TRPV1 ion channel expressed in HEK293 cells assessed as calcium influx by fluo-4-Am-based fluorimetryMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 5nMAssay Description:Agonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as increase in intracellular calcium level by Fluo4-AM based assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 5.30nMAssay Description:Agonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as Ca2+ influx by Fluo-4 dye based assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 5.30nMAssay Description:Agonist activity at human TRPV1 stably transfected in HEK293 cells assessed as increase in calcium influx in presence of ionomycin by Fluo-4-AM dye b...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Binding affinity to human recombinant TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 7.20nMAssay Description:Agonist activity at human TRPV1 expressed in HEK293T cells assessed as increase in Ca2+ transport incubated for 5 mins by calcium-5 fluorescence dye-...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity at human TRPV1 stably transfected in HEK293 cells assessed as decrease in calcium influx preincubated for 5 mins followed by caps...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity at human recombinant TRPV1 expressed in HEK293 cells assessed as desensitization by measuring inhibition of capsaicin-induced int...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity against human recombinant TRPV1 expressed in HEK293 cells assessed as inhibition of capsaicin-induced Ca2+ influx pre-treated 5 m...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Binding affinity to rat TRPV1More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:The effect of the substances on Ca2+ influx was determined by using HEK-293 cells stably overexpressing recombinant human TRPV1 cDNA. The cells were ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Concentration necessary to induce a relative half-maximal response measured by the entry of [Ca2+] into human embryonic kidney HEK293 cells overexpre...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 26nMAssay Description:Agonist activity at human TRPV1 expressed in CHO cells assessed as increase in 45Ca2+ uptake incubated for 5 mins by liquid scintillation counting me...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 29nMAssay Description:Agonist activity at human TRPV1 expressed in HEK293 cells assessed as increase in intracellular calcium accumulation measured at 180 sec by fluoresce...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataKi: 2.00E+4nM ΔG°: -6.41kcal/mole EC50: 29nMpH: 7.4 T: 2°CAssay Description:The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1/2/4(Rat)
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataEC50: 30nMAssay Description:In vitro agonist activity, increased [Ca2+] influx, at vanilloid receptor of rat dorsal root gangliaMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 40nMAssay Description:Agonist activity at human TRPV1 expressed in HEK293 cells assessed as increase in intracellular calcium levelMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 40nMAssay Description:Agonist activity at human recombinant TRPV1 expressed in human HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 44.8nMAssay Description:In vitro [Ca2+] influx relative to capsaicin by Rat Vanilloid receptor (VR1) expressing CHO cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 44.8nMAssay Description:Agonist activity at rat TRPV1 receptor expressed in CHO cells assessed as calcium uptakeMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 45nMAssay Description:Agonist activity at human TRPV1 expressed in CHO cells assessed as stimulation of calcium uptake by 45Ca2+ uptake assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 45nMAssay Description:Agonist activity at rat TRPV1 expressed in CHO cells assessed as increase in 45Ca2+ uptake incubated for 5 mins by liquid scintillation counting meth...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1/2/4(Rat)
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataEC50: 300nMAssay Description:Effective concentration for [Ca2+] uptake into dorsal root ganglia neurones in rat cultured spinal sensory neuronesMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1/2/4(Rat)
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataEC50: 300nMAssay Description:In vitro effective concentration for [Ca2+] uptake into dorsal root ganglia neurones in cultureMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1/2/4(Rat)
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataEC50: 300nMAssay Description:In vitro effective concentration for [Ca2+] uptake into dorsal root ganglia neurones in cultureMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1/2/4(Rat)
Seoul National University
Curated by ChEMBL
Seoul National University
Curated by ChEMBL
Affinity DataEC50: 300nMAssay Description:Compound tested in vitro for [Ca2+] influx into neonatal rat dorsal root ganglia (DRG)More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataIC50: 313nMAssay Description:Activation of human TRPV1 expressed in HEK293F cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataIC50: 420nMAssay Description:Inhibition of TRPV1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of KDM1A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of recombinant LSD1 (unknown origin) using 10-acetyl-3,7-dihydroxyphenoxazine fluorometric substrate measured after 30 mins by multiplate ...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of LSD1 (unknown origin) by KDM1A assay kitMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 870nMAssay Description:Agonist activity at recombinant TRPV1 (unknown origin) expressed in HEK293 cells assessed as induction of calcium flux administered for 1 min followe...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 871nMAssay Description:Agonist activity at recombinant TRPV1 (unknown origin) expressed in HEK293 cells assessed as induction of calcium flux administered for 1 min followe...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily M member 8(Human)
Instituto De Qu£Mica M£Dica
Curated by ChEMBL
Instituto De Qu£Mica M£Dica
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Antagonist activity at TRPM8 (unknown origin)More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataEC50: 1.00E+3nMAssay Description:Agonist activity at TRPV1 (unknown origin) expressed in human SH-SY5Y cells assessed as increase in calcium influx after 25 mins by Fluo-4 NW dye-bas...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataKi: 1.07E+3nMAssay Description:Displacement of [3H]-RTX from human TRPV1 expressed in CHO cells incubated for 60 mins by radioligand competition assay based scintillation counting ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataKi: 1.14E+3nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in CHO cell membranes after 45 mins by scintillation counting methodMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsaicinMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:In vitro binding affinity towards Rat Vanilloid receptor 1 (VR1) by [3H]RTX displacement.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 1.81E+3nMAssay Description:Displacement of [3H]RTX form rat TRPV1 receptor expressed in CHO/VR1 cell systemMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 1.81E+3nMAssay Description:Displacement of [3H]-RTX from rat TRPV1 expressed in CHO cells incubated for 60 mins by radioligand competition assay based scintillation counting me...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Displacement of [3H]RTX from Vanilloid receptor in Rat spinal cord membranesMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rat)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 2.32E+3nMAssay Description:Agonist activity at rat TRPV1 expressed in human SH-SY5Y cells assessed as intracellular calcium accumulation by Fluo-4 NW dye based fluorescence ass...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Human)
Universidad De Antioquia
Curated by ChEMBL
Universidad De Antioquia
Curated by ChEMBL
Affinity DataKi: 2.53E+3nMAssay Description:Displacement of [3H]MPOU from human TRPV1 expressed in CHO cell membranes after 45 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of COX1More data for this Ligand-Target Pair
Affinity DataKi: >5.60E+3nMAssay Description:Displacement of [3H]-CP55940 from human recombinant cannabinoid CB1 receptor expressed in HEK cells by Cheng-Prusoff analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-6.93kcal/molepH: 7.4 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)