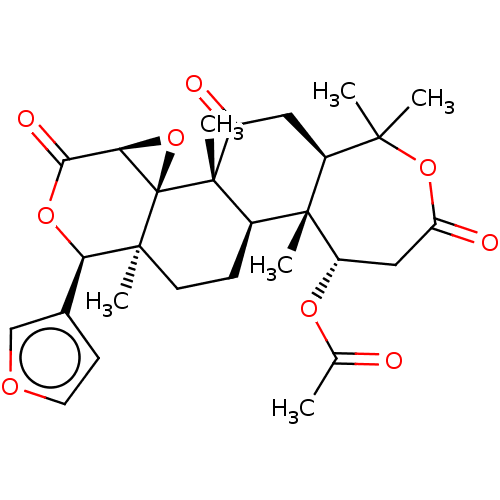
BDBM50480319 Nomilin
SMILES [H][C@]12O[C@@]11[C@@](C)(CC[C@]3([H])[C@@]4(C)[C@H](CC(=O)OC(C)(C)[C@]4([H])CC(=O)[C@@]13C)OC(C)=O)[C@H](OC2=O)c1ccoc1
InChI Key InChIKey=KPDOJFFZKAUIOE-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50480319
Found 3 hits for monomerid = 50480319
TargetReverse transcriptase(Human immunodeficiency virus type 1)
University of Rome Tor Vergata
Curated by ChEMBL
University of Rome Tor Vergata
Curated by ChEMBL
Affinity DataIC50: 6.65nMAssay Description:Inhibition of HIV1 3B reverse transcriptase activityMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus type 1)
University of Rome Tor Vergata
Curated by ChEMBL
University of Rome Tor Vergata
Curated by ChEMBL
Affinity DataIC50: 6.92nMAssay Description:Inhibition of HIV1 3B reverse transcriptase activity infected in human H9 cells assessed as level of p24 antigenMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus type 1)
University of Rome Tor Vergata
Curated by ChEMBL
University of Rome Tor Vergata
Curated by ChEMBL
Affinity DataIC50: 3.88E+5nMAssay Description:Inhibition of HIV1 RTMore data for this Ligand-Target Pair