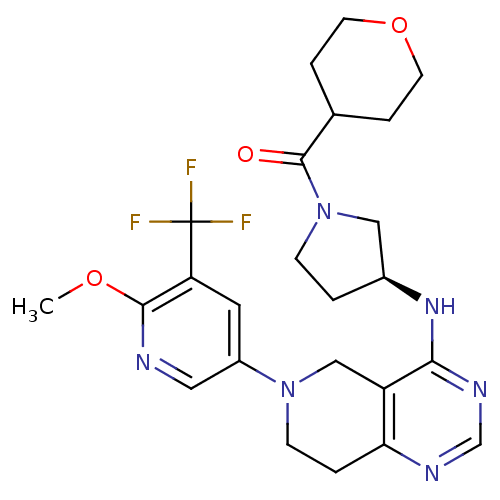
BDBM118300 US8653092, 68
SMILES COc1ncc(cc1C(F)(F)F)N1CCc2ncnc(N[C@H]3CCN(C3)C(=O)C3CCOCC3)c2C1
InChI Key InChIKey=PTFKBGVXOIXYQD-INIZCTEOSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 118300
Found 10 hits for monomerid = 118300
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 43nMAssay Description:Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 7nMAssay Description:Test of Lipid Kinase Activity: The kinase reaction is performed in a final volume of 50 μl per well of a half area COSTAR, 96 well plate. The fi...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of recombinant PI3Kbeta (unknown origin) using phosphatidyl inositol/n-Octyl-glucoside as substrate measured after 60 mins by KinaseGlo as...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.06E+3nMAssay Description:Inhibition GnRH-stimulated luteinizing hormone (LH) release from rat pituitary cellsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of recombinant human myristoylated PI3Kbeta catalytic domain expressed in Rat1 cells assessed as reduction in Akt phosphorylation at Ser47...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 2.15E+3nMAssay Description:Inhibition of recombinant human myristoylated PI3Kalpha catalytic domain expressed in Rat1 cells assessed as reduction in Akt phosphorylation at Ser4...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 4nMAssay Description:Inhibition of PI3Kdelta (unknown origin) using phosphatidyl inositol as substrate measured after 60 mins by Alexa Fluor647-labelled ADP tracer based ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 2.15E+3nMAssay Description:Negative logarithm of the molar concentration of delta receptor was determined in mouse vas deferensMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Mus musculus (Mouse))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of PI3Kdelta in Balb/c mouse splenocytes assessed as reduction in anti-IgM stimulated CD86 expression pretreated for 1 hr followed by anti...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 53nMAssay Description:Inhibition GnRH-stimulated luteinizing hormone (LH) release from rat pituitary cellsMore data for this Ligand-Target Pair