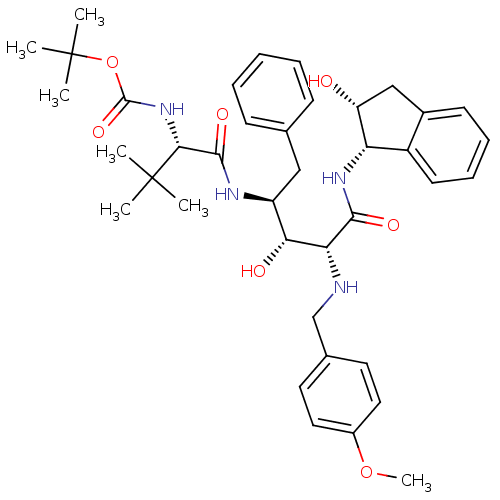
BDBM1212 2-Aminobenzyl-Substituted AHPPA deriv. 28::tert-butyl N-[(1S)-1-{[(2S,3R,4R)-3-hydroxy-4-{[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]carbamoyl}-4-{[(4-methoxyphenyl)methyl]amino}-1-phenylbutan-2-yl]carbamoyl}-2,2-dimethylpropyl]carbamate
SMILES COc1ccc(CN[C@H]([C@H](O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1
InChI Key InChIKey=RVYGXULVCKOQHN-NOMPHJLISA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 1212
Found 2 hits for monomerid = 1212
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Sandoz Forschungsinstitut Ges.M.B.H.
Curated by ChEMBL
Sandoz Forschungsinstitut Ges.M.B.H.
Curated by ChEMBL
Affinity DataKi: 208nMAssay Description:Inhibitory activity was determined against HIV type 1 proteaseMore data for this Ligand-Target Pair
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Sandoz Research Institute
Sandoz Research Institute
Affinity DataKi: 210nMAssay Description:Enzymatic activity was measured by following cleavage of the substrate H-Lys-Ala-Arg-Val-Leu-pNph-Glu-Ala-Nle-NH2. Products of the cleavage reaction ...More data for this Ligand-Target Pair