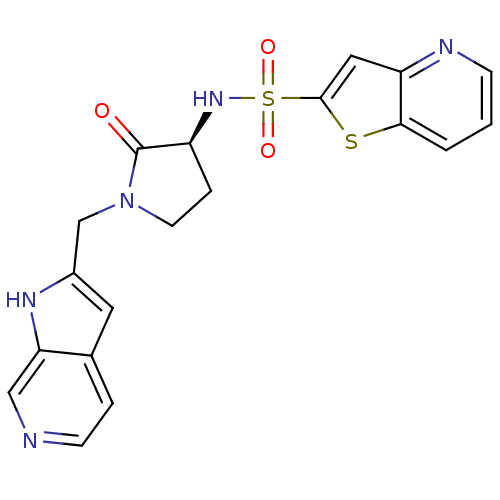
BDBM14057 N-[(3S)-1-(4,7-diazabicyclo[4.3.0]nona-2,4,8,10-tetraen-8-ylmethyl)-2-oxo-pyrrolidin-3-yl]-7-thia-2-azabicyclo[4.3.0]nona-2,4,8,10-tetraene-8-sulfonamide::N-[(3S)-2-oxo-1-{1H-pyrrolo[2,3-c]pyridin-2-ylmethyl}pyrrolidin-3-yl]thieno[3,2-b]pyridine-2-sulfonamide::RPR208707
SMILES O=C1[C@H](CCN1Cc1cc2ccncc2[nH]1)NS(=O)(=O)c1cc2ncccc2s1
InChI Key InChIKey=PLXOQMHGHDZMSX-AWEZNQCLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 14057
Found 4 hits for monomerid = 14057
Affinity DataKi: 18nM ΔG°: -10.5kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: >2.90E+3nM ΔG°: >-7.47kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: >4.00E+3nM ΔG°: >-7.28kcal/molepH: 7.5 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)