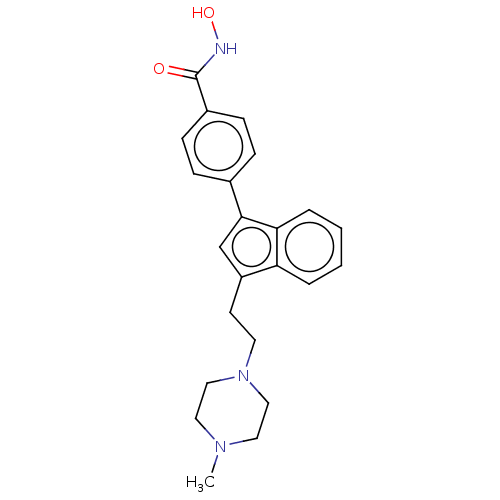
BDBM218230 US9249087, Table 1 , Compound 7
SMILES CN1CCN(CCc2cn(-c3ccc(cc3)C(=O)NO)c3ccccc23)CC1
InChI Key InChIKey=BGQZROPCHZVPNL-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 218230
Found 3 hits for monomerid = 218230
Affinity DataIC50: 2.94nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 174nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 1.23E+3nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair