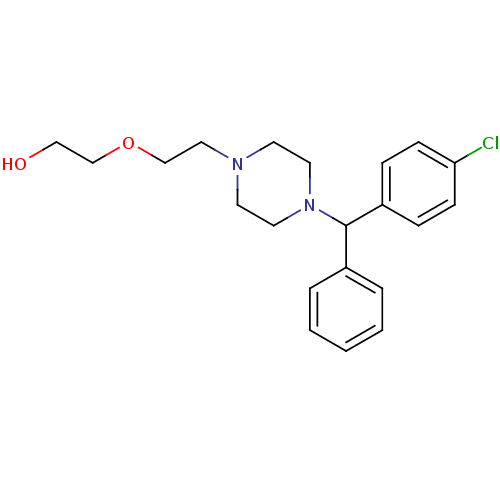
BDBM22875 2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)ethan-1-ol::Atarax::CHEMBL896::Durrax::Hydroxyzine::Hydroxyzine Pamoate::Hydroxyzine, (R)::Orgatrax::Vistaril::med.21724, Compound 187
SMILES OCCOCCN1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1
InChI Key InChIKey=ZQDWXGKKHFNSQK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 22875
Found 10 hits for monomerid = 22875
Affinity DataKi: 2nMAssay Description:Binding affinity to histamine H1 receptor (unknown origin)More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Binding affinity to 5-HT2A receptor (unknown origin)More data for this Ligand-Target Pair
TargetAlpha-1A/Alpha-1B/Alpha-1D adrenergic receptor(Rattus norvegicus (rat))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Displacement of [3H]prazosin from rat cerebral cortex adrenergic receptor alpha1 by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Jagiellonian University Medical College
Curated by ChEMBL
Jagiellonian University Medical College
Curated by ChEMBL
Affinity DataKi: 378nMAssay Description:Binding affinity to D2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-7.09kcal/molepH: 7.4 T: 2°CAssay Description:Ligand displacement assays were performed on The SK-N-MC/hH4R cell homogenates. Retained radioactivity was determined by liquid scintillation countin...More data for this Ligand-Target Pair
Affinity DataEC50: 1.44E+4nMAssay Description:This is a review article.More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+4nMAssay Description:Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea...More data for this Ligand-Target Pair
Affinity DataIC50: 7.29E+4nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair