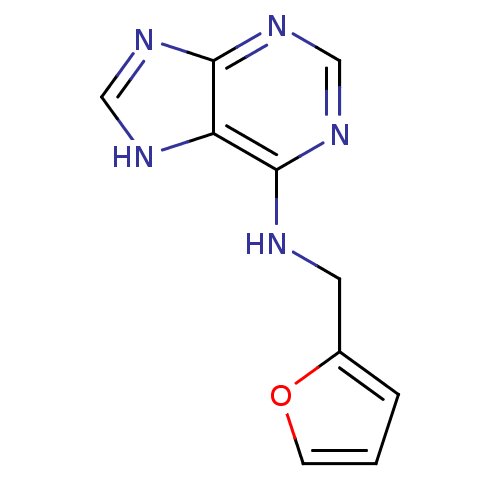
BDBM39302 CHEMBL228792::MLS000101228::N-(2-furanylmethyl)-7H-purin-6-amine::N-(FURAN-2-YLMETHYL)-7H-PURIN-6-AMINE::N-(furan-2-ylmethyl)-9H-purin-6-amine::SMR000017633::cid_3830::kinetin
SMILES C(Nc1ncnc2nc[nH]c12)c1ccco1
InChI Key InChIKey=QANMHLXAZMSUEX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 39302
Found 4 hits for monomerid = 39302
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition of Saccharomyces cerevisiae CTS1More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of human chitinaseMore data for this Ligand-Target Pair
TargetRho-associated protein kinase 2(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 2.14E+4nMAssay Description:Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Center Affiliation: The Scripps Research Institute, TSRI Assay ...More data for this Ligand-Target Pair
TargetEndochitinase A1(Neosartorya fumigata (Aspergillus fumigatus))
University Of Dundee
Curated by ChEMBL
University Of Dundee
Curated by ChEMBL
Affinity DataIC50: >1.00E+6nMAssay Description:Inhibition of Aspergillus fumigatus ChiA1 expressed in Pichia pastoris after 70 minsMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)