BDBM67545 N,N-dimethyl-3-(10-phenothiazinyl)-1-propanamine;hydrochloride::N,N-dimethyl-3-phenothiazin-10-yl-propan-1-amine;hydrochloride::N,N-dimethyl-3-phenothiazin-10-ylpropan-1-amine;hydrochloride::PROMAZINE::SR-01000000228-4::US9504692, Promazine::cid_5887::dimethyl(3-phenothiazin-10-ylpropyl)amine;hydrochloride
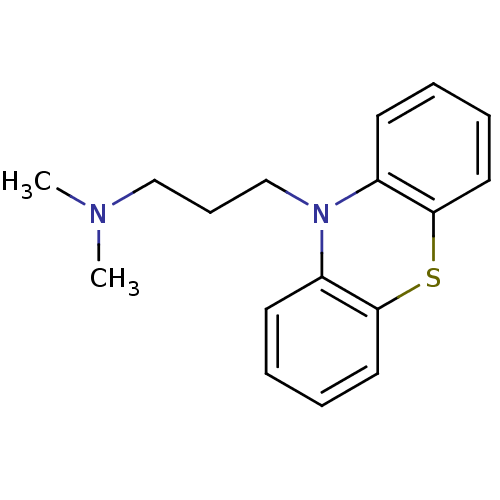
SMILES CN(C)CCCN1c2ccccc2Sc3c1cccc3
InChI Key InChIKey=ZGUGWUXLJSTTMA-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 32 hits for monomerid = 67545
Found 32 hits for monomerid = 67545
Affinity DataIC50: 130nMAssay Description:Compound was tested for inhibition of [3H]spiperone binding in membrane preparations obtained from calf caudate.More data for this Ligand-Target Pair
Affinity DataIC50: 1.04E+3nMAssay Description:Compound was tested for inhibition of [3H]spiperone binding in membrane preparations obtained from rat corpus striatum.More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Displacement of [3H]mepyramine from histamine H1 receptor in Sprague-Dawley rat brain membrane after 2 hr by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.45E+3nMAssay Description:Antagonist activity at H1 receptor in human HeLa cells assessed as inhibition of histamine-induced Ca2+ release by using fura-2AM-based fluorescence ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.71E+3nMAssay Description:Inhibition of recombinant LSD1 (unknown origin) expressed in Escherichia coli BL21 (DE3) using H3K4me2 peptide as substrate incubated for 30 mins by ...More data for this Ligand-Target Pair
Affinity DataEC50: 5.00E+3nMAssay Description:Half maximal inhibition of Prion protein PrPsc formation was assayed in ScN2a cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Mucosa-associated lymphoid tissue lymphoma translocation protein 1(Human)
Helmholtz Munich
US Patent
Helmholtz Munich
US Patent
Affinity DataIC50: 5.80E+3nMT: 2°CAssay Description:To screen for small molecular weight compounds that can inhibit MALT1 protease activity, recombinant GSTMALT1 was purified from E. coli to establish ...More data for this Ligand-Target Pair
TargetPleiotropic ABC efflux transporter of multiple drugs(Baker's yeast)
Wroclaw Medical University
Curated by ChEMBL
Wroclaw Medical University
Curated by ChEMBL
Affinity DataIC50: 1.13E+4nMAssay Description:Inhibition of Pdr5p-mediated rhodamine 6G transport in Saccharomyces cerevisiae MKPDR5h plasma membrane by spectrofluorometric assayMore data for this Ligand-Target Pair
TargetNADPH oxidase 1(Human)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 1.70E+4nMAssay Description:Data Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institut...More data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+4nMAssay Description:Inhibition of 4-(4-(dimethylamino)styryl)-N-methylpyridinium uptake at human OCT1 expressed in HEK293 cells by confocal microscopyMore data for this Ligand-Target Pair
Affinity DataKi: 5.91E+4nMAssay Description:Inhibitory activity against recombinant Trypanosoma cruzi (Trypanosoma cruzi) Trypanothione reductase (linear competitive type)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)