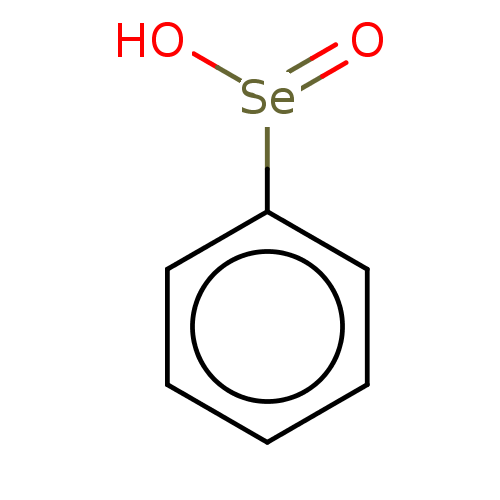
BDBM50028972 Benzene Selenoic Acid
SMILES O[Se](=O)c1ccccc1
InChI Key InChIKey=WIHKGDVGLJJAMC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 50028972
Found 15 hits for monomerid = 50028972
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of recombinant human cytosolic carbonic anhydrase 7 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of recombinant human cytosolic carbonic anhydrase 1 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of recombinant human transmembrane carbonic anhydrase 9 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red ...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of recombinant human cytosolic carbonic anhydrase 2 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red base...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of recombinant human membrane bound carbonic anhydrase 4 pretreated for 15 mins prior to testing measured for 10 to 100 secs by phenol red...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of human BBOX pre-incubated for 25 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of human BBOX pre-incubated for 20 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
Radboud University
Curated by ChEMBL
Radboud University
Curated by ChEMBL
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of wild type recombinant human histone lysine methyltransferase G9a (913 to 1193 residues) expressed in Escherichia coli Rosetta BL21 DE3 ...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT1(Homo sapiens (Human))
Radboud University
Curated by ChEMBL
Radboud University
Curated by ChEMBL
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of wild type recombinant human histone lysine methyltransferase GLP (951 to 1235 residues) expressed in Escherichia coli Rosetta BL21 DE3 ...More data for this Ligand-Target Pair
Affinity DataKd: 1.20E+4nMAssay Description:Binding affinity to human BBOX in presence of Fe(II) by tryptophan fluorescence quenching binding assayMore data for this Ligand-Target Pair
Affinity DataKd: 7.60E+3nMAssay Description:Binding affinity to human BBOX by tryptophan fluorescence quenching binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+4nMAssay Description:Inhibition of human BBOX pre-incubated for 1 min using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of human BBOX pre-incubated for 15 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of human BBOX pre-incubated for 10 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assayMore data for this Ligand-Target Pair