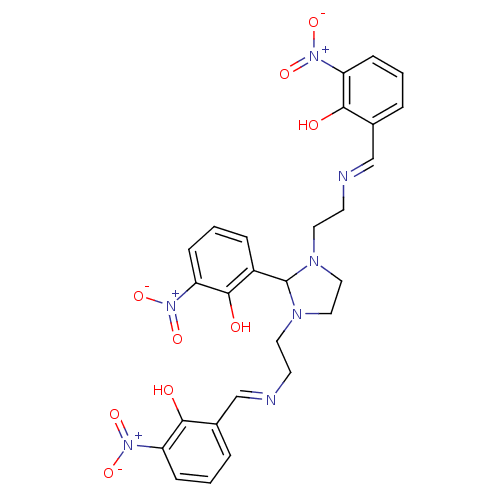
BDBM50056905 2-(1,3-di{2-[1-(2-hydroxy-3-nitrophenyl)-(E)-methylideneamino]ethyl}tetrahydro-1H-2-imidazolyl)-6-nitrophenol::CHEMBL173978::NSC-116625
SMILES Oc1c(\C=N\CCN2CCN(CC\N=C\c3cccc(c3O)[N+]([O-])=O)C2c2cccc(c2O)[N+]([O-])=O)cccc1[N+]([O-])=O
InChI Key InChIKey=XGIUKPGAMWNKBH-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50056905
Found 4 hits for monomerid = 50056905
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 3.67E+4nMAssay Description:Inhibitory concentration of compound against strand transfer of HIV-1 integrase in experiment 1More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 4.95E+4nMAssay Description:Inhibitory concentration of compound against strand transfer of HIV-1 integrase in experiment 2More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 5.27E+4nMAssay Description:Inhibitory concentration of compound against 3'-processing of HIV-1 integrase in experiment 1More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 6.08E+4nMAssay Description:Inhibitory concentration of compound against 3'-processing of HIV-1 integrase in experiment 2More data for this Ligand-Target Pair