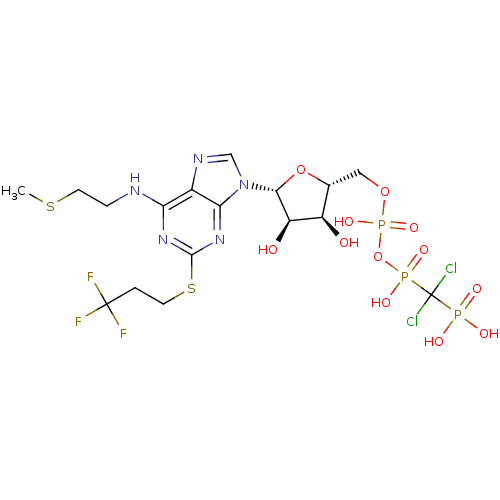
BDBM50118225 ARL 69931MX::Adenosine triphosphate derivative::CHEMBL334966::Cangrelor::US10220040, Compound Reference
SMILES CSCCNc1nc(SCCC(F)(F)F)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O
InChI Key InChIKey=PAEBIVWUMLRPSK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50118225
Found 8 hits for monomerid = 50118225
Affinity DataIC50: 0.398nMAssay Description:Antagonist activity at P2Y12 receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation by turbidimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:The compound was evaluated for antagonist activity against platelet P2Y purinoceptor 12 (P2Y12)More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Antagonist activity at P2Y12 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMAssay Description:Inhibition of P2Y12 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of ADP-induced aggregation preincubated for 5 mins followed by...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Antagonist activity at human platelet P2Y12 receptor assessed as inhibition of ADP-mediated decrease in intra-platelet phosphorylated VASP by FLUO-4 ...More data for this Ligand-Target Pair
Affinity DataEC50: 6.32E+3nMAssay Description:Compounds were assayed for their agonist activity on P2Y13 GPCR transfected cells using a Ca++ flux assay (associated to fluorescent dye detection) w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair