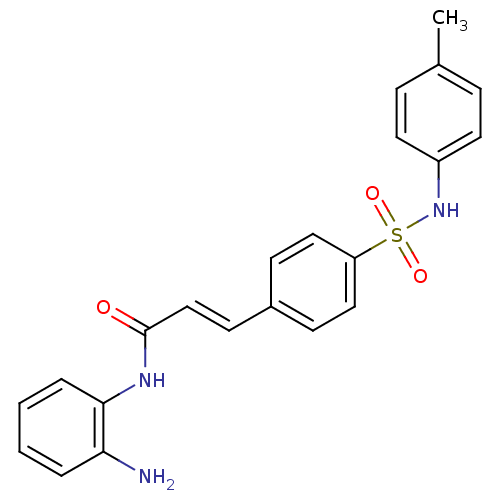
BDBM50123959 (E)-N-(2-Amino-phenyl)-3-(4-p-tolylsulfamoyl-phenyl)-acrylamide::CHEMBL346414::N-(2-Amino-phenyl)-3-(4-p-tolylsulfamoyl-phenyl)-acrylamide::US8796330, 159
SMILES Cc1ccc(NS(=O)(=O)c2ccc(\C=C\C(=O)Nc3ccccc3N)cc2)cc1
InChI Key InChIKey=FVMOHEFQXYYGSP-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50123959
Found 3 hits for monomerid = 50123959
Affinity DataIC50: 1.50E+3nMT: 2°CAssay Description:For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory activity on partially purified recombinant human Histone deacetylase 1 (HDAC-1)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory concentration against human Histone deacetylase 1More data for this Ligand-Target Pair